parkinson’s disease is notoriously difficult to diagnose early and manage effectively. For decades, it’s been understood as a brain disorder, characterized by the devastating loss of dopamine-producing neurons and the buildup of misfolded alpha-synuclein (α-Syn) proteins in the brain. However, a groundbreaking study published in Nature Neuroscience challenges this long-held view, proposing that Parkinson’s might not begin in the brain at all, but in an unexpected peripheral organ: the kidneys.
This discovery could fundamentally change how scientists approach understanding, detecting, and potentially treating this complex neurodegenerative condition. It adds another critical piece to the intricate puzzle of Parkinson’s origins, suggesting the disease could start far from where its most visible symptoms appear.
Understanding Parkinson’s Disease and Alpha-Synuclein
Parkinson’s is known for its motor symptoms like tremors, rigidity, and balance problems. These occur because brain cells vital for movement die off, drastically reducing dopamine levels. At a cellular level, a hallmark of Parkinson’s is the accumulation of abnormal clumps of the protein alpha-synuclein. These misfolded proteins, often called Lewy bodies, disrupt normal brain function and are strongly linked to the disease’s progression.
Traditionally, research focused on why α-Syn begins misfolding and clumping within the brain. But recent theories, like the “gut-brain axis” hypothesis, have suggested the disease might initiate elsewhere in the body and travel to the brain. The new study adds the kidneys to this list of potential starting points.
The Kidney Connection: A Groundbreaking Discovery
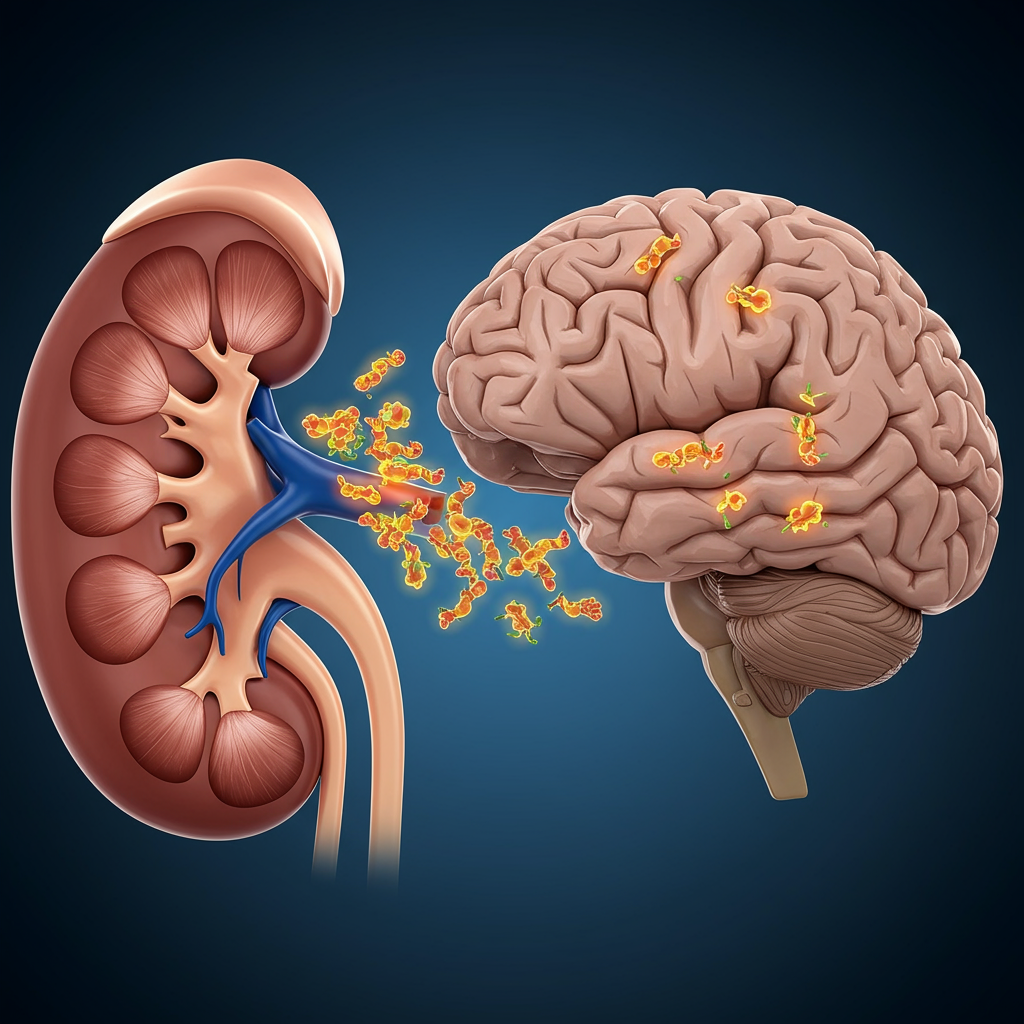
Led by researchers at Wuhan University in China, the study centered on the behavior of the α-Syn protein. Their key finding is that pathogenic α-Syn clumps – the kind associated with Parkinson’s – can originate and accumulate in the kidneys, alongside the brain. The researchers hypothesize that these abnormal proteins may then migrate from the kidneys to the brain, potentially triggering the neurodegenerative process.
“We demonstrate that the kidney is a peripheral organ that serves as an origin of pathological α-Syn,” the research team stated in their published paper. This suggests the kidneys could be a crucial, previously overlooked site in the disease’s pathogenesis.
Evidence from Human Tissue Analysis
To investigate this, the team analyzed human tissue samples. They looked at kidney tissue from individuals with Parkinson’s disease and other forms of Lewy body dementia, as well as samples from people with chronic kidney disease and healthy controls.
A significant finding was the presence of abnormal α-Syn growth in the kidneys of 10 out of 11 people with Parkinson’s or related Lewy body dementias. Even more compellingly, similar protein malfunctions were found in 17 out of 20 patients with chronic kidney disease, despite these individuals showing no signs of neurological disorders at the time. This latter observation is crucial; it provides strong support for the idea that protein clumping can occur in the kidneys before brain damage begins.
Insights from Animal Studies
The researchers also conducted experiments using genetically engineered mice to further test their hypothesis. In mice with healthy kidneys, injected α-Syn clumps were effectively cleared from the body. However, in mice with impaired kidney function, the proteins built up significantly. Crucially, this buildup in the kidneys was followed by the spread of these proteins to the brain.
To explore how the proteins might travel, the team performed additional tests. When the nerves connecting the brain and kidneys were severed in the mouse models, the spread of α-Syn from the kidneys to the brain did not occur. This suggests a potential nerve-mediated pathway for the protein’s migration.
How Could Proteins Travel from Kidneys to Brain?
The study points to at least two possible routes for α-Syn to travel from the kidneys to the brain:
Via Nerves: The experiments involving severing nerves between the kidneys and brain suggest a connection through the nervous system, potentially via the autonomic nervous system.
Via the Bloodstream: The researchers also found that reducing α-Syn levels in the blood correlated with less brain damage in mice. This indicates that α-Syn proteins could also be transported through the circulatory system, offering another pathway for their spread from the kidneys throughout the body, including to the brain.
Understanding these pathways is vital for future research into blocking or interrupting the disease process.
Broader Implications for Understanding and Treatment
This discovery holds significant implications for the future of Parkinson’s research and clinical care. If the kidneys can be an origin point, it opens up new avenues for investigation into:
Early Detection: Could biomarkers for abnormal α-Syn in the kidneys or related blood changes serve as indicators for Parkinson’s risk or early onset, potentially years before motor symptoms appear?
Novel Therapies: Strategies aimed at preventing α-Syn buildup in the kidneys, enhancing kidney clearance of the protein, or blocking its transport from the kidneys to the brain could become new therapeutic approaches. For instance, the researchers suggest that “Removal of α-Syn from the blood may hinder the progression of Parkinson’s disease, providing new strategies for therapeutic management.”
This research reinforces the growing understanding that Parkinson’s is a systemic disease, not confined solely to the brain.
Parkinson’s: A Complex Picture with Multiple Origins?
The idea that Parkinson’s might start in peripheral organs isn’t entirely new. Previous studies have strongly suggested the gut as another potential origin site, with misfolded α-Syn potentially spreading from the digestive system up the vagus nerve to the brain.
The kidney findings, alongside the gut hypothesis, support the notion that Parkinson’s, much like Alzheimer’s disease, is likely triggered by a combination of factors and may originate in different parts of the body in different individuals. This complexity highlights the challenge but also the opportunity for developing multi-pronged prevention and treatment strategies.
Beyond the Kidney: Other Clues to Early Parkinson’s
This kidney study isn’t the only recent research shedding light on early Parkinson’s indicators and risk factors. Scientists are actively exploring various non-motor symptoms that can appear years before typical motor signs.
Early Sensory Changes: Recent work suggests that changes in how the brain processes smell and sight could be early indicators. Studies using advanced brain scanning in mouse models show reduced brain activity in sensory areas in mice engineered to overproduce α-Syn, mirroring early smell loss and visual disturbances seen in many humans with PD. This could potentially lead to new fMRI-based early detection methods in people exhibiting these symptoms.
Dietary Links: Research points to a significant association between diet and Parkinson’s risk. A large study found that high consumption of ultra-processed foods is linked to a higher likelihood of exhibiting multiple prodromal Parkinson’s symptoms, such as sleep disorders, depression, and reduced smell. Conversely, plant-forward diets, like Mediterranean and vegan patterns, show potential benefits. These diets, rich in antioxidants and fiber, may protect against neurodegeneration by reducing inflammation and oxidative stress and supporting a healthy gut microbiome – reinforcing the gut-brain link. High meat consumption, particularly processed meat, has been linked to increased risk and can interfere with common Parkinson’s medications.
- Social Factors: Interestingly, an association has also been reported between loneliness and an increased risk of developing Parkinson’s. While this doesn’t prove causation, it suggests that loneliness might be a marker predicting future disease or could be linked to other underlying factors contributing to risk. It also underscores the known importance of social connection for overall brain health and well-being, particularly for individuals already living with neurological conditions.
- www.sciencealert.com
- www.thebrighterside.news
- neurosciencenews.com
- www.forksoverknives.com
- www.medicalnewstoday.com
These diverse areas of research – from kidney origins to sensory processing, diet, and social factors – paint a picture of Parkinson’s as a disease with multiple entry points and early warning signs, occurring long before its motor manifestations.
Study Limitations and Future Directions
While the kidney study presents exciting possibilities, it’s important to acknowledge its limitations. The number of human tissue samples analyzed was relatively small. Furthermore, while mouse models are valuable scientific tools, findings in animals do not always translate perfectly to humans.
Despite these caveats, the study provides compelling evidence that warrants extensive further investigation. Future research will need to replicate these findings in larger human cohorts, explore the exact mechanisms of α-Syn transport from kidneys to brain in humans, and investigate whether kidney function or markers in the blood could serve as reliable predictors or targets for intervention.
Frequently Asked Questions
What does the new study suggest about where Parkinson’s disease starts?
This study from Wuhan University proposes that Parkinson’s disease might not always start in the brain as traditionally believed. Instead, it suggests that the misfolded alpha-synuclein (α-Syn) proteins associated with the disease can originate and accumulate in the kidneys, a peripheral organ, before potentially spreading to the brain.
What evidence supports the idea that Parkinson’s could start in the kidneys?
The researchers found abnormal α-Syn protein clumps in kidney tissue from people with Parkinson’s and, significantly, in patients with chronic kidney disease who had no neurological symptoms. Animal studies in mice with impaired kidney function also showed α-Syn buildup in the kidneys that then spread to the brain via nerves and possibly the bloodstream.
How do findings about kidney origin relate to other early Parkinson’s research?
The kidney origin theory aligns with other recent research suggesting Parkinson’s can start outside the brain, particularly the gut-brain connection hypothesis. It also complements studies on early non-motor symptoms like smell/sight changes and links between lifestyle factors such as diet (ultra-processed foods, plant-based diets) and loneliness with increased risk or protective effects.
Conclusion
The idea that Parkinson’s disease could originate in the kidneys is a significant shift in our understanding of this challenging condition. This new research, suggesting kidneys as a potential source of pathological alpha-synuclein proteins, opens up exciting avenues for earlier detection methods and entirely new therapeutic strategies that target peripheral organs or protein transport pathways. Combined with ongoing research into other early signs and risk factors like diet and sensory changes, the future of Parkinson’s research is moving towards a more holistic, body-wide view. While more research is needed to confirm and build upon these findings, they offer renewed hope for better ways to prevent, diagnose, and ultimately treat Parkinson’s disease.
Word Count Check: 1118