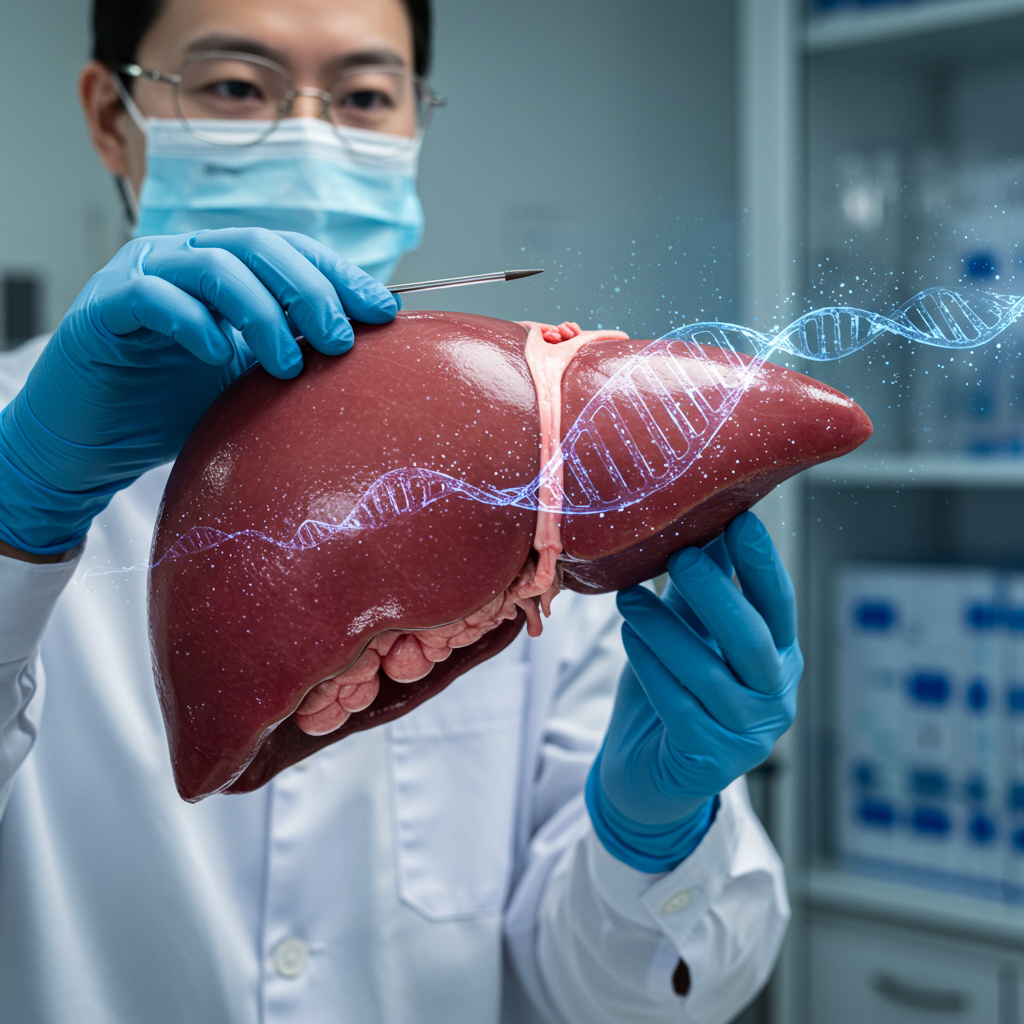
A monumental medical breakthrough recently captivated the scientific community. For the first time, a gene-edited pig liver was transplanted into a living human, offering a glimmer of hope against the critical organ donor shortage. This pioneering procedure, performed by a research team in China, represents a significant leap in xenotransplantation. While the organ functioned for a month, the case highlights both incredible progress and the hurdles still ahead for this innovative field.
The Groundbreaking Procedure: A Lifesaving Attempt
The historic transplant involved a 71-year-old man facing dire circumstances. His liver was severely compromised by irreversible scarring from hepatitis B and cancer. He was ineligible for a conventional human liver transplant. With limited options and time, the patient and his family agreed to an experimental xenotransplant. This compassionate use program took place at a hospital in Anhui Province, China.
The research team, led by surgeon Sun Beiching, implanted an “auxiliary graft” from a genetically modified Diannan miniature pig. This procedure meant the pig liver supported the existing human liver rather than replacing it. Initially, the gene-edited pig liver performed remarkably well. It began secreting bile and carrying out other vital functions once connected to the patient’s circulatory system.
Engineering Compatibility: The Science of Gene Editing
The success of this and other xenotransplants hinges on advanced genetic engineering. Pigs are ideal donors due to their size and anatomical similarities to humans. However, the human immune system naturally rejects animal organs. To overcome this, scientists use tools like CRISPR-Cas9 to modify pig genes.
In similar pioneering studies, six specific genetic edits are often made to pig organs:
Three genes are inactivated: GGTA1, B4GALNT2, and CMAH. These genes produce molecules on pig cells that trigger hyperacute rejection in humans. Removing them reduces the immediate immune attack.
Three human genes are inserted: CD46, CD55, and hTBM. These human genes help protect the pig organ from rejection and prevent dangerous blood clot formation.
These modifications are crucial. They allow the pig organ to better integrate into the human body. Pathogen surveillance also ensures the donor pigs are free from viruses like porcine endogenous retrovirus (PERV) that could harm the recipient.
Early Success and Unforeseen Challenges
For the first month, the gene-edited pig liver graft functioned effectively. This period of successful integration was a major validation for xenotransplantation research. It proved that genetically modified pig livers can support “key metabolic and synthetic functions in humans.” This incredible achievement provided a temporary bridge for the patient.
However, challenges emerged around day 31. Tests revealed a surge in inflammation. Signs of dangerous damage to blood vessels also appeared. This complication was identified as xenotransplantation-associated thrombotic microangiopathy (xTMA). It necessitated the emergency removal of the organ on day 38. While treatment successfully resolved the xTMA injuries, the patient sadly passed away three months later. His death was due to sudden, uncontrollable bleeding in his upper gastrointestinal tract, not directly from the transplant failure itself.
Xenotransplantation: A Broader Horizon for Organ Shortages
This pig liver transplant is part of a rapidly advancing field. Xenotransplantation, the transfer of animal organs to humans, offers hope amidst a dire shortage of human donor organs. Pigs are the most promising donor animals. Their widespread availability and the strides in gene-editing technology make them suitable candidates.
Historically, immune rejection posed an insurmountable barrier. However, modern genetic editing has transformed the landscape. Significant milestones include:
Pig hearts: In 2022, David Bennett received a gene-edited pig heart, surviving for two months.
Pig kidneys: In 2024, Richard Slayman became the first recipient of a gene-edited pig kidney, living for nearly two months. Another patient, Timothy Andrews, has now lived for eight months with a pig kidney.
Pig lungs: Surgeons in Guangzhou, China, performed the first pig-to-human lung transplant in a brain-dead patient in May 2024. The lung showed initial function before complications arose.
These procedures, along with the recent pig liver transplant, demonstrate the growing viability of gene-edited pig tissues for human use.
Expert Perspectives and Future Outlook
Lead investigator Dr. Beicheng Sun described the experiment as a profound success. He stated, “This case proves that a genetically engineered pig liver can function in a human for an extended period.” He believes xenotransplantation can offer a lifesaving bridge for patients. These include those with inoperable liver cancer or untreatable liver failure. His team is now developing pigs with additional genetic modifications. They hope these will lead to even more compatible organs.
Dr. Heiner Wedemeyer, co-editor of the Journal of Hepatology*, hailed the report as a “landmark in hepatology.” He confirmed the pig liver successfully engrafted and delivered essential functions. Dr. Wedemeyer expressed optimism, suggesting that “xenotransplantation may open completely new paths for patients.” These patients include those with acute liver failure or hepatocellular carcinoma. He concluded that “a new era of transplant hepatology has started.”
Despite the enthusiasm, researchers acknowledge remaining hurdles. Coagulation dysregulation and immune complications, including B cell activation, need further optimization. The ongoing research focuses on developing more advanced genetic modifications. Improved immunosuppression regimens are also critical. The goal is to ensure long-term organ function and prevent rejection.
Navigating Ethical Considerations
As xenotransplantation advances, ethical considerations remain paramount. The use of animal organs raises questions about animal welfare and the potential for zoonotic disease transmission. Strict ethical guidelines and comprehensive pathogen surveillance are essential. These measures help ensure patient safety and address societal concerns. Open dialogue among scientists, ethicists, and the public is crucial. This will ensure responsible development of this life-changing technology.
Frequently Asked Questions
What is a gene-edited pig liver transplant, and why is it important?
A gene-edited pig liver transplant involves surgically implanting a liver from a genetically modified pig into a human recipient. This revolutionary procedure is crucial because it offers a potential solution to the severe global shortage of human organ donors. By modifying pig genes, scientists can make their organs more compatible with the human body, reducing the risk of immune rejection. This breakthrough demonstrates the feasibility of using animal organs as a “bridge” therapy or even a long-term solution for patients with end-stage liver disease who have no other options.
How are pig organs genetically modified for human transplant?
Pig organs are genetically modified using advanced techniques like CRISPR-Cas9. Typically, scientists make specific edits to the pig’s genome. They often inactivate genes that produce molecules known to trigger severe immune rejection in humans. At the same time, human genes are inserted into the pig’s DNA. These added genes help regulate the immune system and prevent blood clots within the transplanted organ. These modifications are essential. They allow the pig organ to function safely and effectively within a human body for an extended period.
What are the next steps for xenotransplantation to become a widespread medical option?
For xenotransplantation to become a widespread medical option, several critical steps are necessary. Researchers are focusing on developing even more sophisticated genetic modifications to further enhance organ compatibility and prevent complications like inflammation and blood clotting. Optimizing immunosuppression regimens is also vital to reduce adverse side effects while ensuring the patient’s body accepts the organ long-term. Additionally, extensive clinical trials will be needed to prove safety and efficacy. Addressing ethical considerations and public acceptance will also be crucial for broader implementation of this groundbreaking technology.
The successful first gene-edited pig liver transplant into a living person marks an undeniable turning point. It reignites hope for millions awaiting life-saving organs. While significant challenges persist, the rapid pace of innovation in genetic engineering and surgical techniques points to a future where xenotransplantation could become a routine, life-extending medical practice. The journey continues, fueled by scientific determination and the promise of a world where organ scarcity is no longer a death sentence.