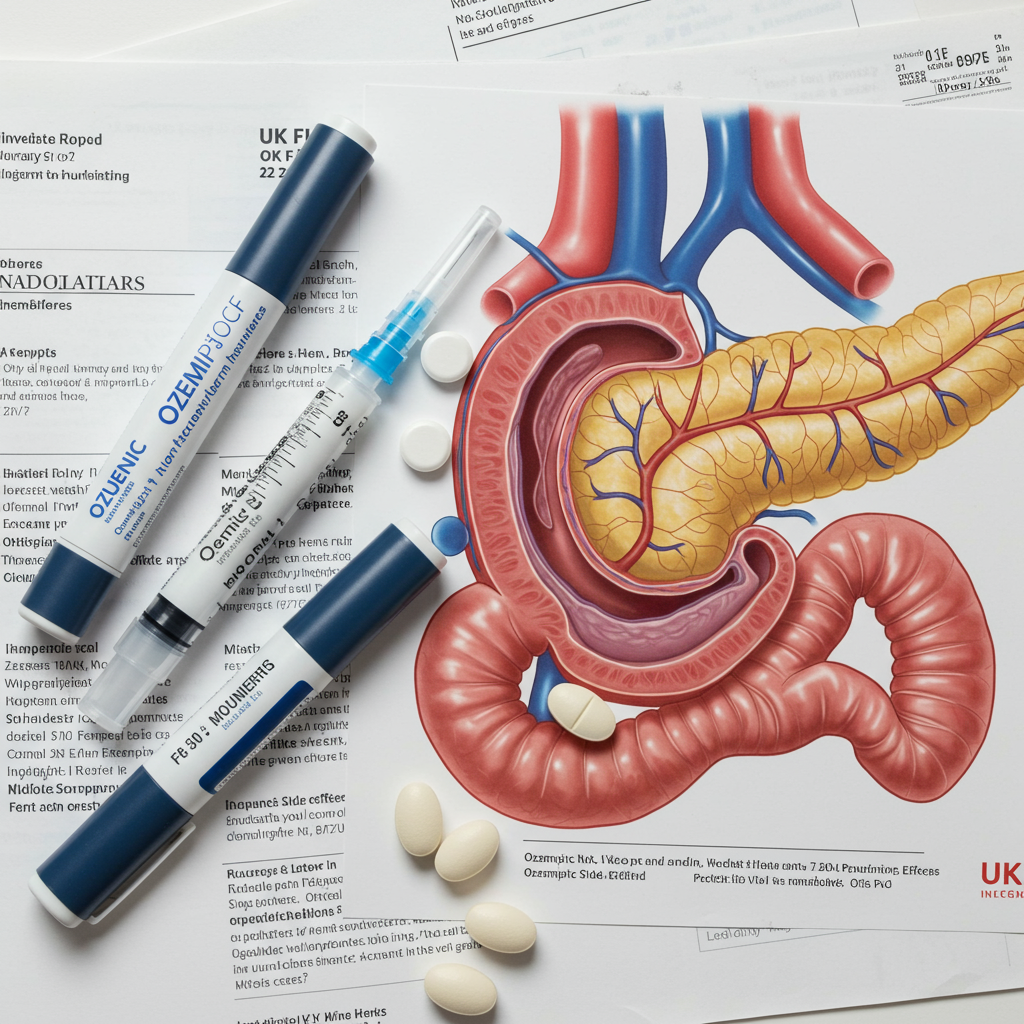
The soaring popularity of GLP-1 medications like Ozempic and Mounjaro, widely used for weight loss and managing type 2 diabetes, is under increased scrutiny in the UK following hundreds of reports of serious pancreatic problems. Health officials have launched a significant investigation into these potential side effects.
Concerns centre on reports of acute pancreatitis, a sudden and severe inflammation of the pancreas, a vital gland for digestion. The Medicines and Healthcare products Regulatory Agency (MHRA), the UK’s health watchdog, has received a growing number of these reports via its Yellow Card scheme, which monitors suspected side effects of medications.
Hundreds of Reports Received
To date, the MHRA has logged nearly 400 reports specifically mentioning acute pancreatitis from individuals using various GLP-1 drugs. While multiple medications are implicated, tirzepatide (sold as Mounjaro) accounts for a substantial portion of these reports, with 181 cases linked to the drug. Other widely used medications like semaglutide (Ozempic, Wegovy) have also seen significant reports, with 113 cases for acute and chronic pancreatitis combined.
While acute pancreatitis is listed as an “uncommon” side effect in patient information leaflets – potentially affecting up to 1 in 100 people – the volume of reports is rising alongside the increasing number of people using these drugs across the UK, estimated to be around 1.5 to 1.6 million.
Serious Risks, Including Reported Fatalities
Acute pancreatitis is a serious condition often requiring hospitalisation due to symptoms like severe abdominal pain, nausea, and fever. Tragically, some reports submitted to the Yellow Card scheme involving GLP-1 medicines and pancreatitis have been associated with fatalities. Across various GLP-1 drugs, reports have mentioned 10 deaths where pancreatitis was suspected. It is crucial to understand that these Yellow Card submissions represent suspected links reported by patients or healthcare professionals and do not definitively confirm the medication as the sole cause; other factors, including pre-existing health conditions or even obesity itself (which is a risk factor for pancreatitis), could play a role.
UK Regulator Launches Genetic Study
In light of the severity of pancreatitis and the increasing reports, the MHRA is taking action. They have launched a major study, the Yellow Card Biobank project, in partnership with Genomics England.
The primary goal of this pioneering study is to explore whether an individual’s genetic makeup might influence their susceptibility to developing serious side effects, particularly acute pancreatitis, when taking GLP-1 medications.
Experts note that adverse drug reactions are a significant issue, accounting for one in six hospital admissions and costing the NHS billions annually (£2.2bn+ in hospital stays alone). Understanding genetic predispositions could be key to preventing many adverse reactions. Dr. Alison Cave, the MHRA’s chief safety officer, highlighted that genetic testing has the potential to prevent almost a third of medicine side effects, paving the way for safer, more personalised medicine.
Patients who have been hospitalised with suspected acute pancreatitis linked to using a GLP-1 medicine are strongly encouraged to report their experience via the MHRA’s Yellow Card scheme. Those who report may be invited to participate further in the Biobank study, providing additional health information and a saliva sample for genetic analysis.
Manufacturer Perspectives
Drug manufacturers are also addressing the concerns. Lilly, the maker of Mounjaro, stated that patient safety is paramount and they actively monitor and evaluate safety data. They noted that while they take all reports seriously, adverse events can have various causes, including other health factors, and highlighted that their product leaflet already lists acute pancreatitis as an uncommon side effect and advises caution for those with a history of the condition.
Similarly, Novo Nordisk UK, which produces Ozempic and Wegovy, emphasised patient safety and that the known risks and benefits are detailed in product information. They recommend using the medications only for approved indications under strict healthcare professional supervision and assert that the benefit-risk profile of their GLP-1 medicines remains positive. Both companies expressed openness to research that improves the understanding of treatments.
While GLP-1 drugs are seen as valuable tools in managing conditions like obesity and type 2 diabetes, health officials caution they are not a universal fix and carry known risks. Beyond the focus on pancreatitis, common side effects include gastrointestinal issues like nausea and constipation, and the MHRA recently warned that Mounjaro might reduce the effectiveness of oral contraceptive pills in some users.
The ongoing investigation and the new genetic study represent a crucial step in understanding and potentially mitigating the risks associated with these widely used medications, aiming for safer prescribing practices tailored to individual patient profiles.