In a landmark move positioning the UK at the forefront of cancer innovation, the NHS in England is set to offer a revolutionary new blood cancer therapy described as a “Trojan horse.” This pioneering treatment, known as belantamab mafodotin (also referred to as Blenrep), is now available for eligible patients battling multiple myeloma, an incurable cancer affecting plasma cells in the bone marrow.
The therapy represents a significant advancement, offering hope to patients whose multiple myeloma has returned or stopped responding to previous treatments. Clinical trials have demonstrated remarkable results, showing that when used in combination with other standard therapies like bortezomib and dexamethasone, it can keep the cancer at bay for an average of three years. This is a dramatic improvement compared to the typical progression-free period of just over one year seen with some commonly used existing treatments.
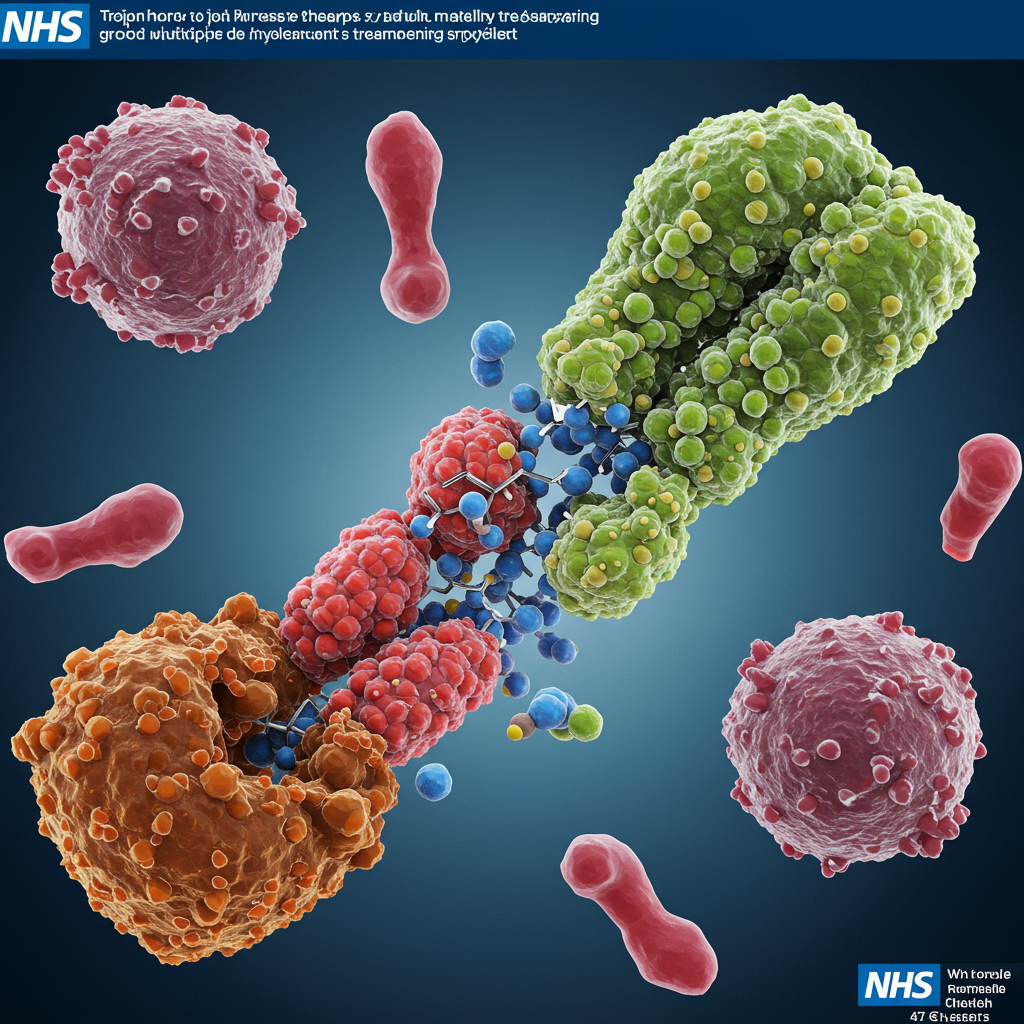
How the “Trojan Horse” Therapy Works
Belantamab mafodotin is a sophisticated type of medicine called an antibody-drug conjugate (ADC). The “Trojan horse” analogy comes from its ingenious mechanism:
A targeted antibody is designed to specifically seek out and attach to markers found on the surface of cancerous plasma cells.
Once bound, the therapy is absorbed inside the cancer cell.
- Inside the cell, it releases a potent, cell-killing chemotherapy drug directly to the source, effectively destroying the cancer from within while minimizing damage to healthy cells elsewhere in the body.
- www.bbc.com
- www.theguardian.com
- www.lbc.co.uk
- conservativepost.co.uk
- www.bbc.com
This targeted delivery allows for a much higher concentration of the toxic drug precisely where it’s needed, potentially reducing the severe side effects often associated with conventional chemotherapy.
Life-Changing Impact for Patients
For those living with relapsed multiple myeloma, this therapy offers a crucial lifeline. Approximately 1,500 patients in England each year are expected to benefit. The National Institute for Health and Care Excellence (NICE) has recommended the drug as cost-effective for NHS use, and it is being funded via the Cancer Drugs Fund to ensure fast-tracked access.
The impact is already being felt. Paul Silvester, a 60-year-old patient from Sheffield, received the therapy through an early access programme after his myeloma relapsed following a bone marrow transplant. Describing the experience as “amazing” and “absolutely life-changing,” Paul achieved remission within weeks of his first dose. He now enjoys a “good normal life” and is busy planning history-themed adventures, such as visiting Hadrian’s Wall, and looking forward to his daughter’s graduation.
Professor Peter Johnson, NHS England’s national clinical director for cancer, hailed the drug as a “game-changer,” emphasizing its potential to provide patients with years more precious time with friends and family, free from the debilitating symptoms of the disease.
A Step Towards a Functional Cure
While multiple myeloma is currently considered incurable, experts view drugs like belantamab mafodotin as crucial steps forward. Professor Martin Kaiser, team leader in myeloma molecular therapy at the Institute of Cancer Research, called these ADCs “very smart drugs” with a “remarkable” difference in side effects compared to some other treatments. He believes such therapies are “an important step towards a functional cure,” potentially pushing long-term remission rates above 50% in the next five years.
UK-Led Innovation
This groundbreaking therapy has strong roots in the UK. Developed by GSK, early research took place at their facility in Stevenage, with the first clinical trials conducted on UK patients in London. This journey from discovery to national rollout highlights the strength of Britain’s life sciences sector and the effective collaboration between science, the NHS, and pharmaceutical innovation. Antoine Herbaux, Head of Oncology UK at GSK, noted this approval as a significant milestone showcasing local innovation leading to positive patient outcomes.
Important Considerations
While kinder than some other treatments, belantamab mafodotin is not without side effects. The most common include dry eyes and blurred vision, which occur because residual drug can leak into the body after cancer cells are destroyed. Patients receiving the therapy require routine eye examinations before and during treatment. The drug is administered via infusion, typically every three weeks when used in combination with other drugs.
Wider Impact
The success of antibody-drug conjugates in treating myeloma is also paving the way for their development in combating other cancers. Research is already underway for their use in certain types of breast, stomach, and bowel cancers. However, the challenge lies in designing antibodies that can precisely target only cancer cells.
Patient advocacy groups have strongly supported the drug’s approval. Shelagh McKinlay, Director of Research and Advocacy at the charity Myeloma UK, called the NHS becoming the first globally to roll out the drug “fantastic,” stating it would “transform the lives of thousands” and celebrating the UK’s position at the forefront of myeloma treatment. Health Minister Karin Smyth echoed these sentiments, calling it a “groundbreaking therapy” that positions the NHS at the forefront of cancer innovation.
The availability of belantamab mafodotin marks a significant moment in the treatment of relapsed multiple myeloma, offering extended remission and improved quality of life through its innovative, targeted approach.