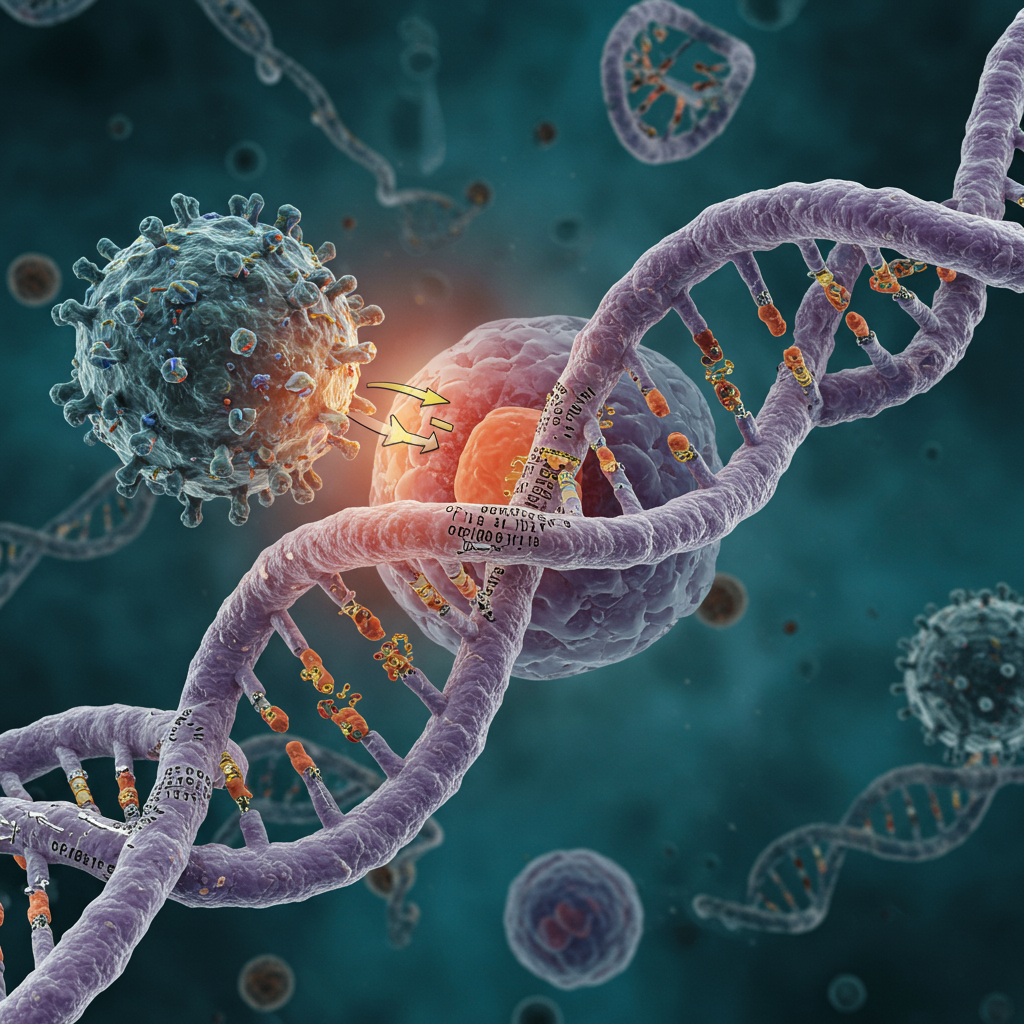
Experiencing a cold sore isn’t just an uncomfortable nuisance; it’s a sign that the herpes simplex virus type 1 (HSV-1) is actively hijacking your body’s most fundamental machinery. New research has revealed a remarkable and slightly unsettling truth: this common virus begins to dramatically reshape our genetic material within mere hours of infection, strategically manipulating it to fuel its own replication and spread.
Found in billions worldwide, HSV-1 often lies dormant but can become active, typically causing painful sores around the mouth. It spreads easily through close contact. When active, the virus invades host cells, making its way to the nucleus – the cell’s control center where our DNA resides.
The Virus’s Swift Takeover
Our DNA is typically wound around proteins, forming structures within the nucleus. Some sections are tightly coiled, effectively switching genes off, while others form looser loops, allowing genes to be active and transcribed into molecules needed for cell function. Prior studies had observed that HSV-1 infection led to tighter coiling of the host genome, potentially disrupting normal cellular processes, but the exact mechanism was unclear.
Scientists at the Centre for Genomic Regulation in Barcelona, Spain, led by researchers like Alvaro Castells-Garcia and Esther González-Almela, sought to understand this process better. Using high-resolution microscopy to observe HSV-1 infecting human lung cells, they gained an unprecedented view of the viral interaction with our DNA.
Their findings, published in Nature Communications, were striking. Within just one hour of entering a cell, the virus had already begun to disrupt the host genome. They confirmed previous observations that HSV-1 steals essential transcription proteins – the very molecules needed to unwind and read DNA sequences – away from the human genome.
Strategic DNA Manipulation
Crucially, the study uncovered why the host DNA coils up so tightly. This widespread coiling, causing the genome to shrink to a mere 30 percent of its original volume within eight hours, is a direct result of the virus siphoning off these vital transcription proteins. Without these proteins readily available, the DNA structure defaults to a more compact, tightly wound state.
But the virus doesn’t just cause random chaos. The researchers discovered that HSV-1 is highly strategic. It makes contact with specific regions of the host genome, not just any random part. These targeted regions are precisely those that contain the genes encoding the very transcription proteins the virus needs and is stealing.
Targeting Genes for Viral Gain
By interacting with these specific sites, the virus prevents them from coiling up like the rest of the genome. Even more cunningly, it actively boosts the activity of genes within these targeted regions. Why? Because these are the genes critical for producing the components the virus needs to replicate – its own RNA and proteins. This targeted manipulation ensures a continuous supply of resources necessary for the infection cycle to continue, demonstrating a sophisticated level of viral control over host cellular processes.
“We used to think it made contact with our genome randomly,” says team member Esther González-Almela. “But the virus is able to contact our own genome in specific regions, and these regions usually harbour genes that are involved in the continuity of infection, in making viral RNA and proteins.”
Hope for New Treatments
Understanding this intricate manipulation opens doors for potential new antiviral strategies. In a promising part of the study, the researchers tested an experimental cancer drug known to block one of the transcription proteins that HSV-1 steals. They found that this drug successfully prevented the cold sore virus from replicating in human lung cells in the laboratory.
This suggests that therapies targeting the virus’s ability to steal and manipulate these host proteins could be effective, particularly for individuals facing severe HSV-1 infections or those with weakened immune systems who are more vulnerable to complications. While milder cases often resolve on their own, more potent treatments are needed for serious instances.
Experts, like Benjamin Krishna at the University of Cambridge, note that other viruses similar to HSV-1, such as those causing chickenpox (varicella zoster virus) or even common colds (adenoviruses), might employ similar tactics to hijack host machinery. This research, therefore, holds potential implications beyond just cold sores, suggesting these types of experimental drugs could perhaps work against a wider range of viral infections.
This groundbreaking study not only sheds light on the incredibly swift and strategic ways HSV-1 co-opts our cells but also offers a compelling new avenue for developing targeted antiviral therapies that could benefit millions.