Sometimes sugar isn’t just a simple treat; in the complex world of biology, it can be a powerful, even dangerous, molecule. We often hear about the negative health impacts of excessive sugar intake, contributing to conditions like obesity, diabetes, and cardiovascular problems by promoting chronic inflammation and increasing fat accumulation (as research from Harvard indicates). One way this happens is through a process called glycation, where surplus sugars, specifically reducing sugars, spontaneously attach to vital proteins in the body, potentially disrupting their function.
Normally, our bodies have mechanisms to counteract this. An enzyme called fructosamine 3-kinase, or FN3K, plays a key role in breaking down these damaging glycated proteins. For decades, FN3K’s function was known, but detailed information about its precise atomic-level structure, crucial for developing targeted therapies, remained elusive.
But what if glycation wasn’t always the enemy? In a fascinating twist being explored by cancer researchers, glycation can sometimes work against a tumor. Certain proteins are essential for cancer cells to grow and spread. Tumors are greedy for sugar to fuel this rapid growth, and in some cases, this excess sugar can spontaneously attach to these growth-promoting proteins via glycation. This attachment can actually “stuff the mouth” of the protein, dampening its activity and hindering the tumor’s ability to proliferate.
In such scenarios, the body’s natural cleanup crew, FN3K, becomes counterproductive because it would remove this beneficial glycation that is inhibiting tumor growth. Therefore, scientists are exploring a bold new strategy: inhibiting FN3K to preserve the glycation that keeps the tumor in check.
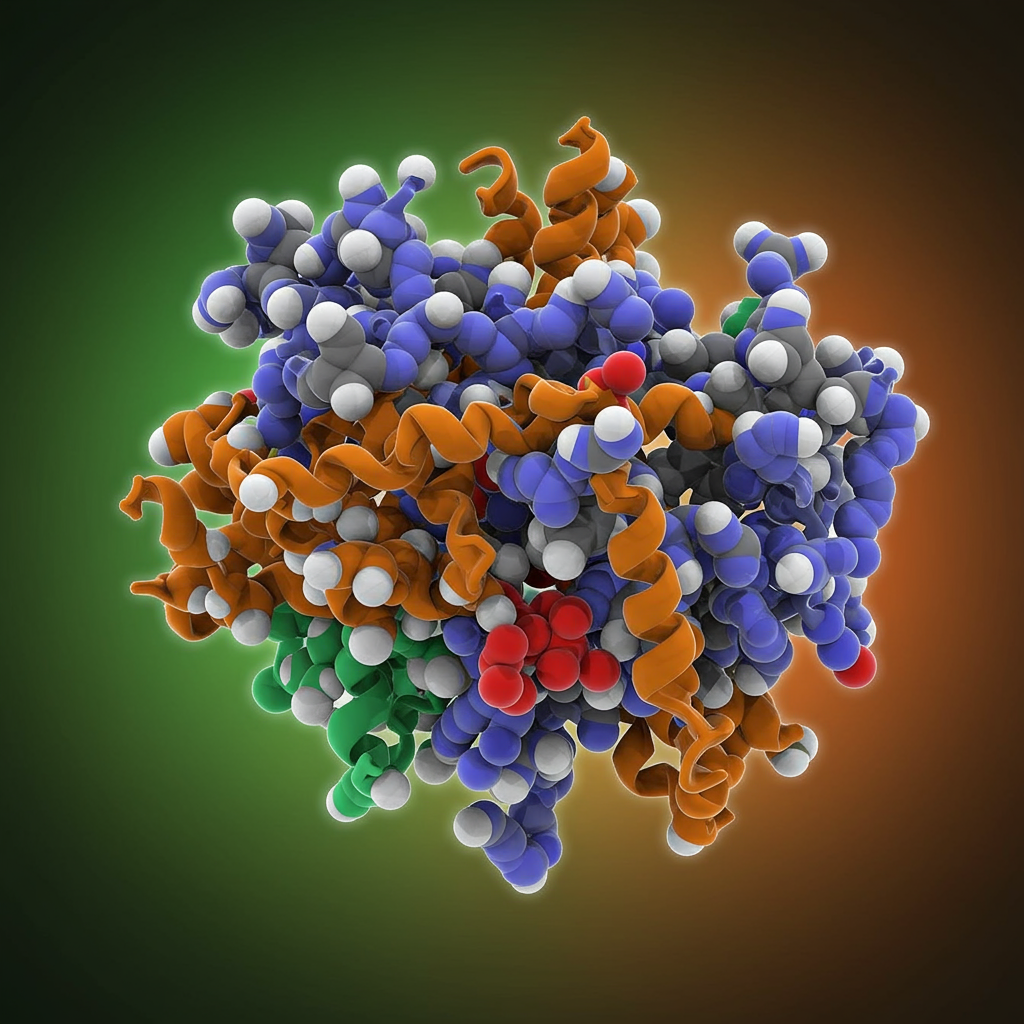
This is where groundbreaking research from Cold Spring Harbor Laboratory (CSHL) comes in. Led by Professor Leemor Joshua-Tor and with key contributions from postdoc Ankur Garg, a team has, for the first time, revealed the complete 3D structures of human FN3K in different functional states.
Understanding the precise 3D structure of an enzyme like FN3K is paramount for drug development. Kinases are common throughout the body, and any potential drug targeting FN3K must be highly specific to avoid interfering with other essential cellular processes. As Professor Joshua-Tor explains, having a precise model “gives us a precise model” to guide this specificity.
Using these newly unveiled structures, the CSHL team meticulously mapped out the entire process by which human FN3K breaks down glycated substrates. They made a remarkable discovery: unlike most other kinases, human FN3K contains a unique amino acid – tryptophan. When the researchers removed this tryptophan, the enzyme became inactive. Even more surprisingly, changing this single amino acid to a different one transformed FN3K into a “super-enzyme.” This highlights how precisely tuned these biological machines are and that controlling even one tiny component can dramatically alter the enzyme’s function.
This detailed understanding of FN3K’s structure and the critical role of amino acids like tryptophan provides a clear roadmap for therapeutic development. Collaborators at Memorial Sloan Kettering Cancer Center are already utilizing this data to design potential drug candidates aimed at inhibiting FN3K. While clinical applications are still a long-term goal, the immediate next step involves identifying existing small molecules that can bind to FN3K based on its newly understood structure.
This research into targeting specific enzymes like FN3K adds another dimension to the multifaceted fight against cancer. It complements other areas of research exploring how broader factors like diet and metabolism influence cancer progression. Studies, such as those at the University of Rochester Medical Center, have shown that adopting a plant-based diet can improve metabolic markers like weight, cholesterol, and levels of growth factors like IGF-1 in patients with advanced cancer. Excess weight, high IGF-1, and inflammation are all factors known to contribute to cancer risk and progression, supporting the idea that influencing the body’s metabolic environment through diet can impact disease outcomes.
Indeed, research highlights that a balanced diet rich in fruits, vegetables, whole grains, legumes, and healthy fats may play a role in reducing cancer risk and supporting health during treatment, due to beneficial compounds like antioxidants, fiber, and specific plant nutrients. This suggests that while molecular research targets specific pathways like FN3K, lifestyle factors like diet address the broader systemic environment in which cancer exists.
The revelation of the FN3K structure represents a significant step forward in understanding a potentially exploitable pathway in cancer metabolism. By providing a precise target, this discovery opens up new avenues for developing highly specific therapies that could leverage the cancer cell’s own sugar dependence against it, offering fresh hope in the ongoing battle against this deadly disease.