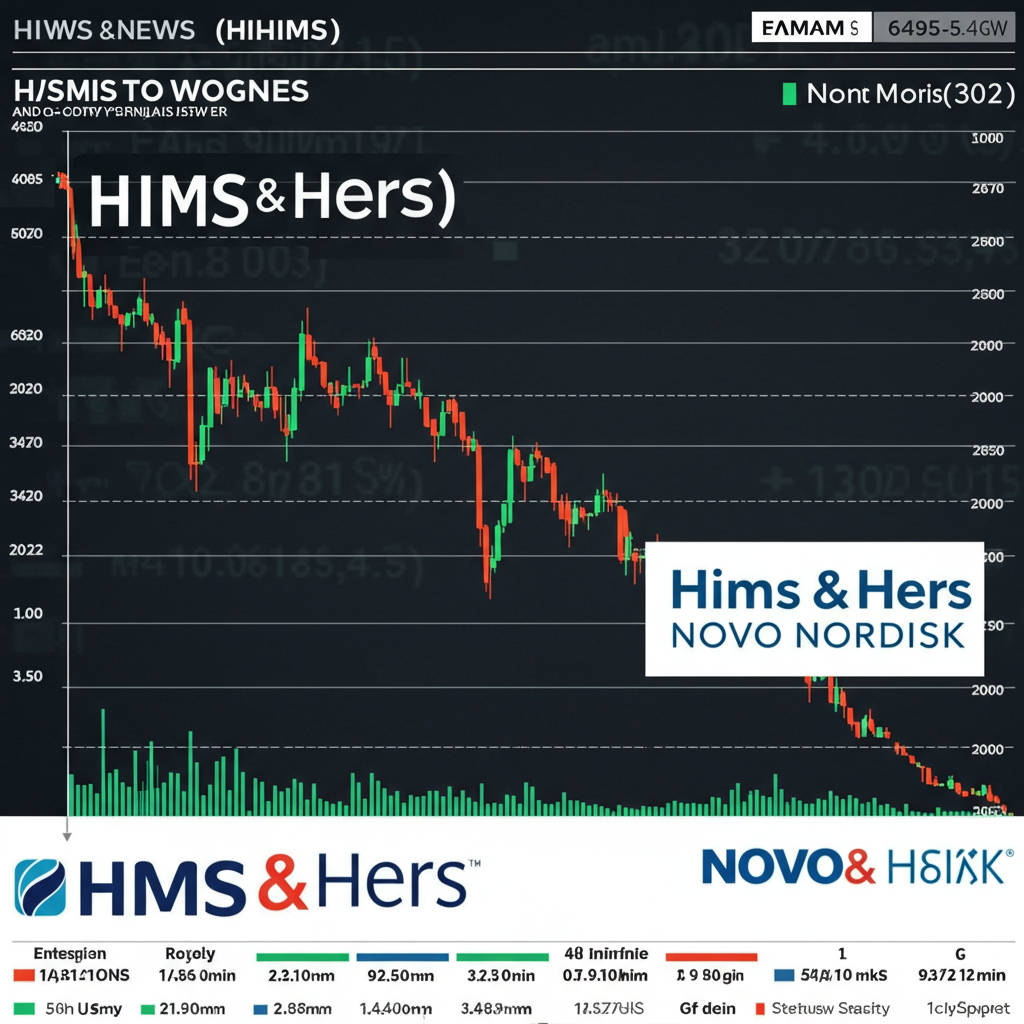
Hims & Hers (HIMS) shares experienced a dramatic sell-off today, plunging approximately 30%, following pharmaceutical giant Novo Nordisk’s decision to terminate its collaboration deal. The partnership, which aimed to provide patients direct access to Novo Nordisk’s popular weight-loss injection, Wegovy®, was abruptly ended, with Novo Nordisk citing significant concerns over patient safety and Hims & Hers’ operational practices.
Why Novo Nordisk Pulled the Plug
The collaboration, announced just over a month prior, was intended to help transition patients who had previously used compounded (copycat) versions of semaglutide (Wegovy’s active ingredient) during widespread shortages to the authentic, FDA-approved drug. However, Novo Nordisk stated it terminated the agreement because Hims & Hers allegedly failed to comply with laws prohibiting the mass sale of compounded drugs under the guise of “personalization.”
Novo Nordisk’s statement was explicit, accusing Hims & Hers of engaging in “illegal mass compounding” and disseminating “deceptive marketing” for what it deemed “illegitimate, knockoff versions of Wegovy®.” The company expressed particular alarm regarding the source of the “semaglutide” active pharmaceutical ingredients (APIs) used in these compounded drugs, stating that based on its investigation, they are often manufactured by foreign suppliers in China. Novo Nordisk highlighted that the FDA has reportedly not authorized or approved the manufacturing processes or reviewed the quality of “semaglutide” from many of these foreign suppliers, asserting that U.S. patients should not be exposed to drugs made with such potentially unsafe and illicit ingredients.
Dave Moore, Executive Vice President, US Operations of Novo Nordisk Inc., reinforced the company’s stance on patient protection, emphasizing that individuals prescribed semaglutide are entitled to authentic, FDA-approved Wegovy®. He stated that Novo Nordisk would continue to take action against companies engaged in “illegal sham compounding” that jeopardizes public health, and will continue to work with select telehealth organizations that share their commitment to patient safety.
Hims & Hers Responds
While Hims & Hers did not have an immediate public response to media inquiries following the announcement, CEO Andrew Dudum later addressed the situation on social media. Dudum reportedly claimed that Novo Nordisk’s commercial team had pressured Hims & Hers to “steer patients to Wegovy,” and that the company refused to be “strong-armed” by what he termed “anticompetitive demands” that limit provider decision-making and patient choice.
The context around compounded GLP-1 drugs is complex. While compounding pharmacies were permitted to produce copycat versions during the severe shortages of branded drugs like Wegovy and Eli Lilly’s Mounjaro (tirzepatide), this allowance has legal nuances. The FDA generally permits compounding for individual patient needs (e.g., allergies, dosage adjustments) under a “personalization” loophole, but Novo Nordisk’s claim focuses on alleged mass sales under this guise. Reports indicate that Eli Lilly is also facing similar issues with some telehealth platforms continuing to offer compounded products.
Market Impact and Analyst Sentiment
The loss of the Wegovy partnership is seen as a significant blow to Hims & Hers, particularly as entry into the burgeoning weight-loss market was considered a major growth catalyst. Shares plummeted, contributing to a year-to-date decline from previous highs.
Adding to investor jitters, Hims & Hers had recently provided weaker-than-expected guidance for its second-quarter sales, forecasting revenue below analyst expectations. This follows a notable deceleration in overall subscription growth year-over-year.
On TipRanks, HIMS currently holds a “Hold” consensus rating based on a mix of 2 Buy, 7 Hold, and 2 Sell ratings. The average analyst price target of $42.09 suggests a potential downside of over 9% from recent trading levels. However, some analysts are more bearish. Bank of America, for instance, maintains a “Sell” rating on HIMS shares with a $28 price target, implying a potentially significant further decline from current levels. Their caution stems from the loss of the Wegovy opportunity, the associated reputational damage, and potential regulatory scrutiny related to compounding practices, alongside existing financial headwinds.
Novo Nordisk’s U.S.-listed shares (NVO) also saw a decline following the news, though less severe than HIMS.
Challenges and Opportunities
Beyond the Wegovy fallout, Hims & Hers faces other potential challenges. A new Federal Trade Commission (FTC) rule set to take effect in July could simplify subscription cancellations, potentially increasing customer churn. The company also faces potential pressure from Congress and government bodies regarding direct-to-consumer marketing of pharmaceuticals, having previously drawn criticism for advertising compounded GLP-1s during the Super Bowl.
Despite these hurdles, some analysts point to other potential growth areas for Hims & Hers. Hormone Replacement Therapy (HRT) is identified as a lucrative market opportunity, estimated between $3 billion and $10 billion, which could add meaningful revenue in the coming year. Hims & Hers has a track record of quickly scaling new product categories, and its direct-to-consumer model and brand recognition could help expand the addressable market for HRT over time.
In summary, the termination of the Novo Nordisk partnership over serious safety allegations regarding compounded drugs presents significant challenges for Hims & Hers, impacting its growth trajectory in the weight-loss market and potentially increasing regulatory scrutiny. While other opportunities like HRT exist, the immediate focus remains on the financial and reputational fallout from the Wegovy deal’s collapse, leading many analysts to adopt a cautious stance on the stock.