The future of food production and clean energy could hinge on a single, essential chemical: ammonia. This compound, made of nitrogen and hydrogen, is the cornerstone of fertilizers that feed roughly half the world. It’s also emerging as a promising carbon-free fuel and carrier for clean hydrogen energy.
However, producing ammonia today comes at a staggering environmental cost. The dominant method, known as the Haber-Bosch process, relies heavily on fossil fuels, consuming immense energy and releasing significant amounts of carbon dioxide. This single industrial process accounts for 1-2 percent of global carbon emissions. Scientists globally are racing to find a cleaner, more sustainable way to create this vital chemical.
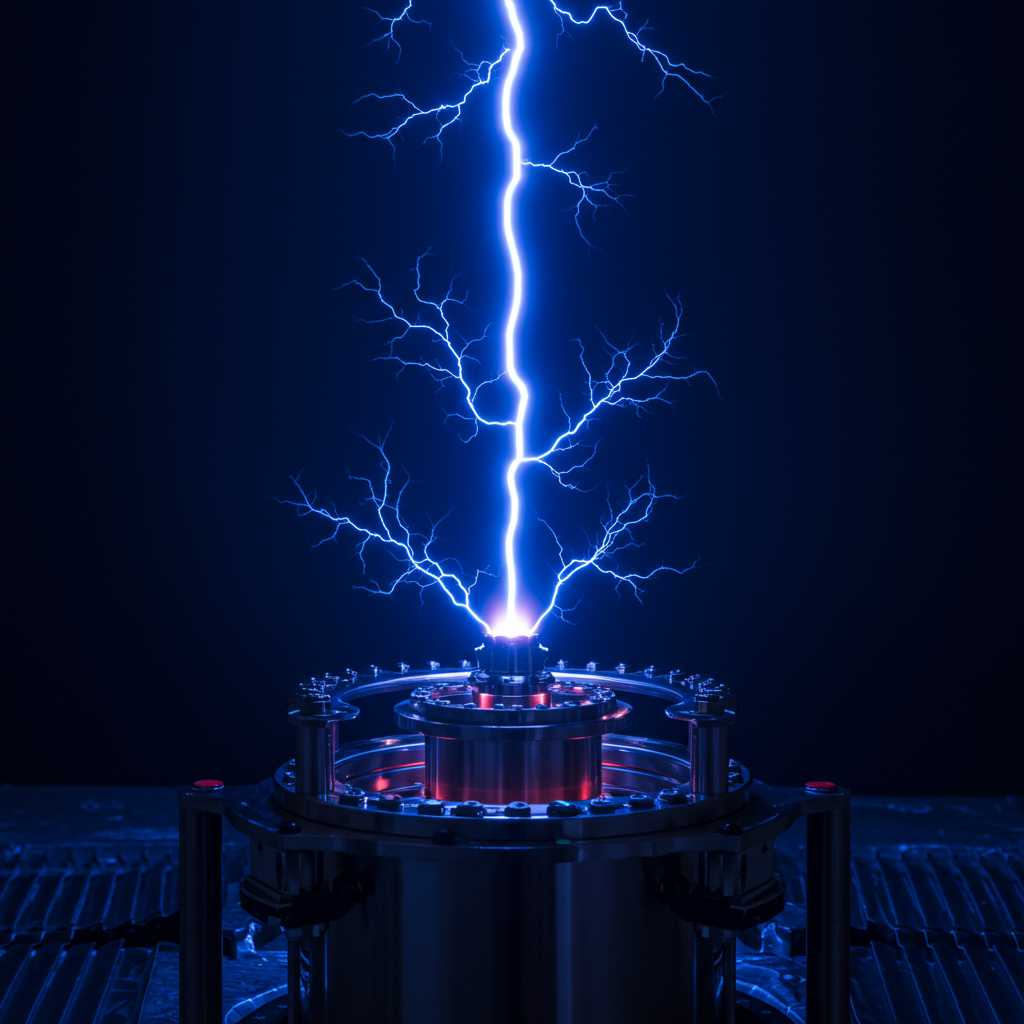
Revolutionizing Ammonia Production with ‘Human-Made Lightning’
Researchers at the University of Sydney have developed a novel method that could dramatically change how ammonia is made. Led by Professor PJ Cullen, their team has harnessed the power of plasma, effectively creating “human-made lightning,” to convert atmospheric air into ammonia gas. This breakthrough offers a potentially low-carbon, decentralized alternative to the energy-intensive Haber-Bosch process.
The traditional Haber-Bosch method requires combining nitrogen and hydrogen gases under extreme heat and pressure, using a catalyst. This process demands centralized, large-scale facilities typically located near cheap natural gas sources, which provides the hydrogen and energy. The Sydney team’s approach bypasses many of these requirements.
A Two-Step Pathway to Green Ammonia
The innovative process developed by Professor Cullen’s team involves two main steps:
- Plasma Excitation: Using electricity, the researchers excite nitrogen and oxygen molecules found abundantly in ordinary air. This creates a plasma state – similar to a tiny lightning bolt or the phenomenon seen when zapping air bubbles in water. This energetic step is crucial for breaking the strong triple bond of nitrogen molecules, a significant challenge in ammonia synthesis.
- Electrolyzer Conversion: The excited nitrogen and oxygen molecules are then directed to a specialized membrane-based electrolyzer containing a catalyst. Within this ‘silver box,’ hydrogen is added, converting the excited molecules directly into ammonia gas (NH₃).
A key advantage of this method is that it produces ammonia in its gaseous form immediately. Some previous alternative techniques resulted in ammonia in a liquid solution (ammonium, NH₄⁺), which requires additional energy-intensive steps to become the usable gas.
Why Sustainable Ammonia Is Critical
The demand for ammonia is continuously rising, driven primarily by the need for fertilizer to support a growing global population. Before industrial synthesis, societies relied on natural sources like guano (bird droppings), which was so valuable it even led to historical conflicts. The invention of Haber-Bosch in the early 20th century revolutionized agriculture, making massive food production possible and sustaining billions.
But the climate impact of this success is undeniable. A sustainable, “green” ammonia that doesn’t depend on fossil fuels is urgently needed. This new method, powered potentially by renewable electricity, offers a pathway to drastically reduce the carbon footprint associated with ammonia production.
Decentralizing Production
Unlike the massive, centralized plants required for Haber-Bosch, a plasma-based method could potentially be scaled down. This could enable smaller, perhaps even farm-scale, production of ammonia for fertilizer. Such decentralization would eliminate the significant energy and emissions associated with transporting large quantities of ammonia over long distances.
Beyond Fertilizer: Fueling the Future
The potential applications of green ammonia extend far beyond agriculture. With three hydrogen atoms per molecule, ammonia is being seriously considered as an efficient carrier for transporting hydrogen energy. Renewable hydrogen could be converted into ammonia, shipped globally, and then ‘cracked’ back into hydrogen at its destination.
Furthermore, ammonia is a strong candidate for a carbon-free fuel itself. When burned, ideally, it produces only nitrogen and water, making it attractive for decarbonizing hard-to-abate sectors like long-haul shipping. The shipping industry alone accounts for about 3 percent of global greenhouse gas emissions, and transitioning to fuels like green ammonia is seen as vital for meeting climate goals.
Challenges and Next Steps
While the Sydney team’s research represents a significant leap forward, challenges remain. The plasma step of their two-step process is reportedly already energy-efficient and scalable. However, the electrolyzer component needs further improvement. Professor Cullen notes that enhancing the energy efficiency of the electrolyzer is the current focus to make the overall method economically competitive with the established, albeit polluting, Haber-Bosch process. Experts suggest the electrolyzer needs to be about three times more efficient to be industrially viable.
Developing green ammonia technologies is an active area of research worldwide, exploring various approaches, including improving electrolysis and other electrochemical methods. Achieving cost parity with fossil fuel-based ammonia is crucial for widespread adoption, potentially requiring supportive policies and infrastructure development.
This Sydney University research, published in Angewandte Chemie International Edition, highlights the promise of harnessing electrical methods to fix nitrogen from the air. It underscores the global scientific effort to move towards a sustainable chemical industry essential for feeding the world and powering a clean energy future.
Frequently Asked Questions
What is “green ammonia” and why is it needed?
Green ammonia is ammonia (NH₃) produced using renewable energy sources, significantly reducing or eliminating the carbon emissions associated with its production. It’s urgently needed because the traditional method, the Haber-Bosch process, is highly energy-intensive, relies on fossil fuels, and contributes 1-2 percent of global CO2 emissions. Green ammonia is vital for decarbonizing agriculture (fertilizer), becoming a clean fuel, and serving as a carrier for renewable hydrogen, supporting the transition to a sustainable economy.
How does the new Sydney University method work differently from the traditional Haber-Bosch process?
The University of Sydney’s new method is a two-step process that uses electricity to convert air directly into ammonia gas. It starts by creating plasma (‘human-made lightning’) to excite and break down nitrogen and oxygen molecules from the air. These excited molecules then pass through a membrane-based electrolyzer where hydrogen is added to form gaseous ammonia. In contrast, the Haber-Bosch process combines nitrogen and hydrogen gases under extremely high temperatures and pressures using a catalyst, typically requiring significant energy from fossil fuels and operating in large, centralized plants.
What are the potential uses for ammonia produced by this new method?
Ammonia produced by this potentially low-carbon method has multiple critical applications. Primarily, it can be used to create sustainable fertilizers essential for global food production, potentially enabling decentralized, on-site manufacturing for farmers. Beyond agriculture, ammonia is being explored as a carbon-free fuel, particularly for hard-to-electrify sectors like shipping, and as an efficient chemical carrier for transporting renewable hydrogen energy across distances.